
Our laboratory consists of three research groups led by Dr Urania Georgopoulou (Research director), Dr Pelagia Foka (Senior researcher) and Dr Athanassios Kakkanas (Specific Operations Scientist Grade A’). Research at the HPI Molecular Virology Laboratory is dedicated to the study of viruses, covering a wide range of topics, including: cloning and study of viral genomes and genetic variability, structure and function of viral proteins, virus – host interactions, viral persistence, mechanisms of infection, antiviral immunity and immunometabolism, virus-directed intercellular communication, pathobiology of viral diseases, oncogenic viruses, virus-triggered autoimmune and neurodegenerative diseases, identification of therapeutic targets against viral diseases and the development of prophylactic / medical countermeasures against emerging and re-emerging viral infections Lately, we have undertaken studies on the development of “more physiologically-relevant” tissue environments for the study of viral infection pathobiology by constructing and validating novel in vitro 2D and 3D cell culture and co-culture models. Viral families currently under investigation include, but are not limited to, members of the Flaviviridae, Hepadnaviridae, Coronaviridae, Herpesviridae and other viral families.
Exciting new basic and applied research projects involving exosomes, key intercellular transporters of host and medicinal cargo, as well as various virological and other nanoparticle applications, have been initiated by our team, utilising the NanoSight NS300 nanoparticle analyser, which was purchased with funds from the institutional Regional Operational Programme (PEP Attikis).
1. Viral and Host Determinants Shape the Outcome of Viral Infection and Persistence
Viruses are highly diverse microorganisms. Their heterogeneous genetic and structural features, together with a battery of diverse replication strategies and infection mechanisms, ensure evolvability and survival. The biological diversity exhibited by RNA viruses is a combination of their short generation times, fast evolutionary rates and rapid adaptation against selective pressures imposed by evolving antiviral therapies, the environment, hosts and their immune system. On the other hand, DNA viruses are believed to have more stable genomes, however, this notion can be widely challenged by single-stranded or smaller DNA viral species. Overall, this wondrous genetic polyphony, is often matched by equivalent host genetic determinants, resulting in a well-defined symbiosis between virus and the host immune system, or, if left unchallenged, leads to viral persistence and disease manifestation.
Projects in this thematic area involve:
- Biochemical Analysis of structural and regulatory features of SARS-CoV and SARS-CoV-2 viral proteins and associated host factors (lead researchers: P. Foka, A. Kakkanas)
- Establishment of novel in vitro cell culture infectious models for the study of HBV / HDV ((lead researchers: U. Georgopoulou)
- HCV YXXΦ motifs, viral entry and establishment of infection (lead researchers: U. Georgopoulou, E. Karamichali)
- Defective Viral Genomes of HCV / HBV and Liver Disease Progression (lead researchers: U. Georgopoulou, E. Karamichali)
- Host Genetic Polymorphisms and Persistence of Hepatitis Viral Infections (lead researchers: U. Georgopoulou, P. Foka)
- The effect of HCV core genetic variability in the activation of pro-tumourigenic molecular mechanisms (lead researcher: A. Kakkanas)
-Establishment of cell culture propagation of the animal BVDV and study of signal transduction changes during infection (lead researchers: U. Georgopoulou, P. Foka)
2. Dissecting the Metabolic, Epigenetic and Signalling Mechanisms implicated in Virus-Host Interactions and the Progression to Virus-related Cancer
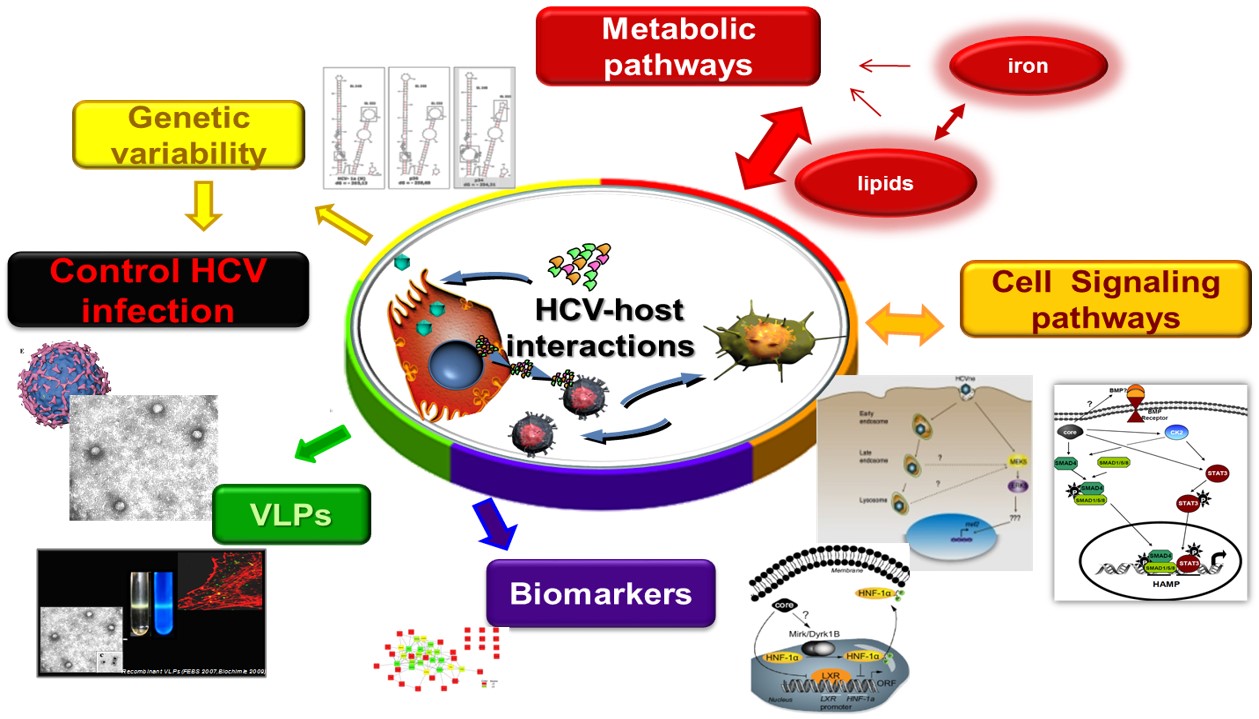
Hepatitis C (HCV) and B virus (HBV) infections often lead to severe liver disease, including cirrhosis and hepatocellular carcinoma (HCC). HCC accounts for approximately 90% of the incidence of all primary liver cancers, and it is the 5th most prevalent cancer worldwide and the 4th leading cause of death globally. Recently, direct-acting anti-viral (DAAs) therapies have achieved to eliminate HCV in the majority of cases. However, low barriers to resistance-associated mutations, reduced efficacy in the treatment of difficult patient groups, inability to prevent HCV-induced HCC after viral eradication, recurrence of HCV or latent HBV post-treatment and the lack of an effective vaccine, all underscore the need for better understanding of (a) the viral biology and persistence, (b) host-virus interactions, (c) monitoring disease progression and HCC onset through the identification of molecular signatures/biomarkers so that (d) novel therapies are discovered.
Projects in this thematic area involve:
- HCV Infection and Host Epigenetic and Pro-metastatic Factors in HCC Development and Progression (lead researchers: U. Georgopoulou, P. Foka)
- The Role of Host Lipid Metabolism in HCV-associated HCC (lead researcher: P. Foka)
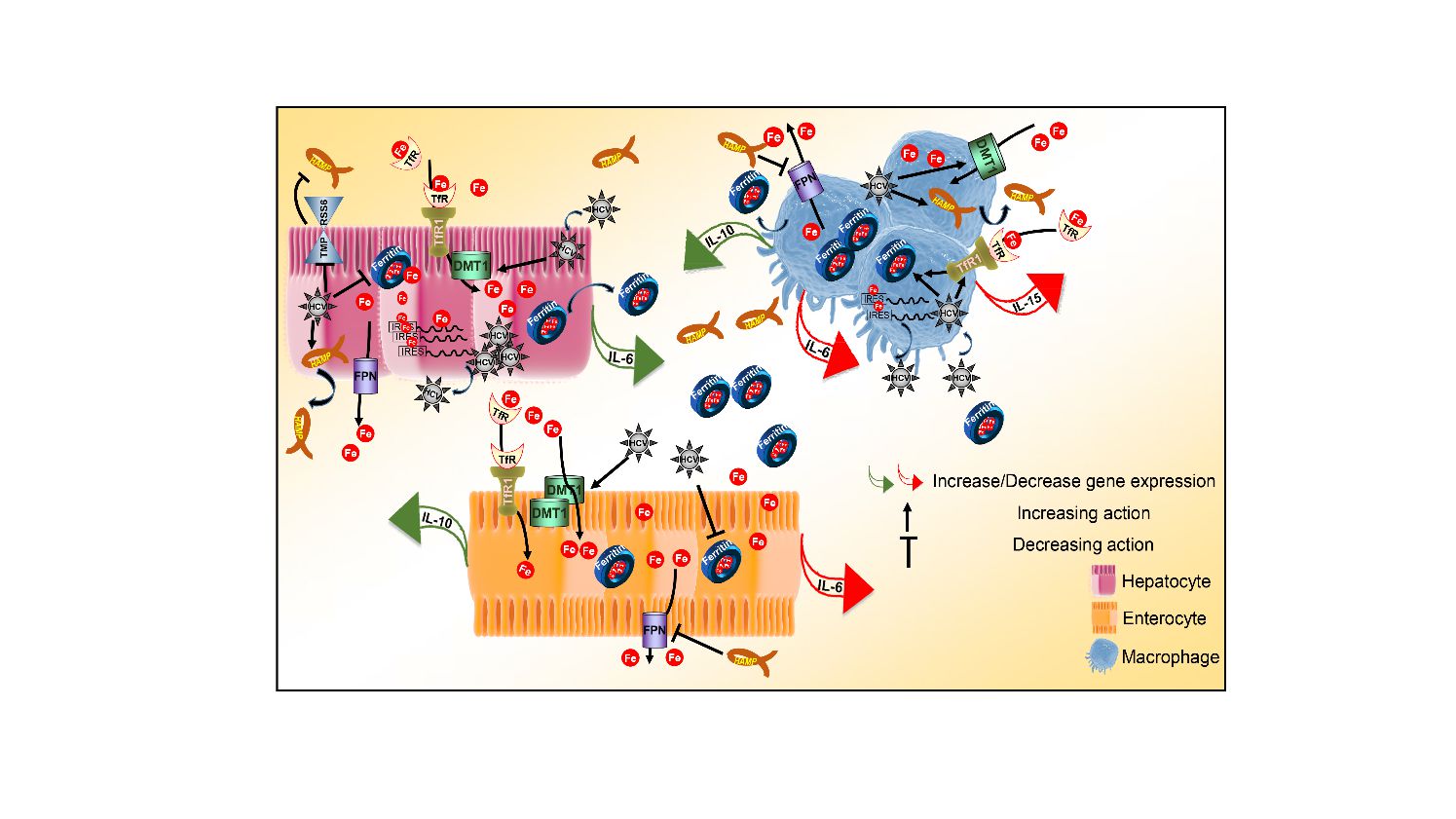
- The Role of Host Iron Homeostasis in HCV infection and Persistence (lead researchers: U. Georgopoulou, P. Foka)
- Analysis of Protein Interaction Networks for the Discovery of Hepatitis B and C Biomarkers (lead researchers: U. Georgopoulou, A. Kakkanas)
3. Viral Infection, Viral Persistence and the Host Immune Response
Depending on whether the virus is eliminated by the immune system, or not, viral infections are either acute or chronic. Chronic infections are further classified into latent infections, where the virus is dormant, but occasional viral replication may occur during periodic episodes of reactivation (e.g. HSV-1), or persistent, where viral replication continues to maintain persistent viraemia (e.g. HCV, HBV). The outcome of a viral infection depends on the antiviral immune responses of the host.
Deregulated host innate immune responses fail to eliminate the virus during the acute phase, thereby facilitating the establishment of viral chronicity. Innate immune mechanisms, including the activation of TLR-mediated antiviral pathways are deployed in the presence of viral nucleic acids, leading to IFN type- and cell-specific transcriptional gene regulation. Infected-cell killing by NKCs and viral antigen-presenting by DCs are both instrumental for the early innate immune responses. Monocytes, infiltrating macrophages from the periphery and tissue-resident macrophages also hold key roles in viral infection, including viral antigen presentation, production of pro- and anti-inflammatory cytokines/chemokines and induction of T-cell activity.
Chronic persistent infections are typically associated with compromised adaptive immune responses. CD4+ T-cells, and CD8+ T-cells play a significant antiviral role by balancing the combat against viral pathogens and the risk of developing autoimmunity or overwhelming inflammation. Tregs have also been shown to play an important role in the attenuation of antiviral T-cell responses and immune-mediated host injury and inflammation, through CD8+ T-cell inhibition in both the acute and chronic phases.
Recent findings suggest that immune responses may be modulated by changes in cellular metabolic pathways that can effectively alter the function of immune cells of both adaptive and innate immunity.
Projects in this thematic area involve:
- Cell-mediated immunity in Viral Hepatitis (Lead researchers: E. Karamichali, U. Georgopoulou)
- SARS-CoV-2 and host immune responses: The role of bioactive lipids and host immunoreceptors (Lead researcher: P. Foka)
- Iron Homeostasis and Innate Immune Responses in HCV Infection ((Lead researcher: P. Foka)
4. Exosomes: The masters of intercellular communicators in viral infection, health and disease
Exosomes are nanosized extracellular vesicles that are formed intracellularly by fusion of late endosomes with the plasma membrane. Once released from the parental cells, they can travel large distances and attach to recipient cells through endosome-derived components and surface molecules. Initially, they were thought to be cellular “waste bins”, however, during the last decade, exosomes have been identified as important mediators of intercellular communication in normal physiology and disease. They carry and transfer diverse cargo of cell-derived biomolecules, including proteins, mRNAs, miRNAs, lipids and other metabolites from one cell to another, thereby shuttling information both in an autocrine and endocrine manner. Exosomal contents reflect the status of parental cells, hence the vivid interest on their potential use as biomarkers of disease. Exosomes play a crucial role in viral infections, as vehicles of viral genomes and components. They shelter viruses, assisting them to avoid immune detection. In addition, virus-related exosomal cargo enables de novo infection and completion of viral life cycles.
Our laboratory has developed a vivid interest in exosome biogenesis and biological function, as well as researching their nanocarrier properties.
Projects in this thematic area involve:
- The impact of exosomal cargo in the immune response of chronic HCV patients after treatment with Direct-Acting Antivirals (Lead researchers: E. Karamichali, U. Georgopoulou)
- Detection of exosomal neurodegeneration markers in the aftermath of SARS-CoV-2 infection (Lead researchers: E. Karamichali, P. Foka)
5. Viruses as triggers of neurodegeneration
An increasing body of evidence suggests that viruses may act as triggers of complex molecular events that eventually lead to neurodegeneration. Grave human diseases, such as Parkinson’s (PD) and Alzheimer’s (AD) disease have been associated with viral infections or reactivation of dormant viruses. Major human viruses, such as Hepatitis C virus, Herpes simplex virus-1, Varicella-zoster virus, West Nile virus, Epstein-Barr virus, Influenza virus, Human Immunodeficiency virus and now SARS-CoV-2, have all been cited as risk factors for PD and AD development or neurodegenerative-like syndromes. The key pathophysiological processes by which viruses contribute to the development of such disorders remain unclear. It is possible that a combination of pre-existing genetic predisposition, environmental factors and recurring viral infections may initiate the cascade of molecular events that builds up to neurodegeneration and its pathology, mainly through neuroinflammatory effects, mitochondrial dysfunction, oxidative stress, direct neurotoxicity and the deregulation of the brain-dedicated immune cells.
Projects in this thematic area involve:
- SARS-CoV-2-triggered Cognitive Decline and Neurodegeneration Mechanisms in the COVID-19 aftermath (Lead researchers: P. Foka and E. Karamichali)
- Innate immunity and neuroinflammation in SARS-CoV-2, HSV-1 and Influenza infections (Lead researcher: P. Foka)
6. Development of molecular / immuno-diagnostic tools and prophylactic measures for the detection of and protection against respiratory, arboviruses, hepatitis and herpes viruses
![]()
Various tools for molecular diagnostics and immunodiagonostics, as well as study of viral properties are designed and constructed in our laboratory. Viral capsids are the first well-defined, multiprotein supra-molecular biostructures encountered by a naive host cell. Thus, they have evolved to contain all necessary means for engaging and docking the host cell, as well as propelling the viral genetic material into the cytoplasm, so that commencement of the viral cycle occurs. Synthetic virus-like particles (VLPs), or pseudocapsids, carry many characteristics of parental viruses that can be harnessed in vaccine development strategies. In fact, several VLP-based vaccines are now commercially available, including vaccines against the carcinogenic Human Papilloma Virus (HPV) and Hepatitis B Virus (HBV). In parallel, pseudocapsid-based biological tools are able to provide a versatile platform to screen libraries for entry inhibitors and to evaluate neutralising antibody responses. On the other hand, pseudotyped viruses, based on the VSV, lenti- or retrovirus platforms enable the surface expression of viral capsid glycoproteins, independently of biosafety status, thereby allowing the entry requirements of many highly pathogenic viruses. Such heterologous systems can also be used to complement VLPs in the rational design and validation of medical countermeasures, diagnostics and novel surface materials that exhibit antiviral properties.
Projects in this thematic area involve:

- Integrating Multi-Disciplinary Expertise in a Learning and Adaptive European Pandemic Preparedness System: Development and validation of effective medical countermeasures that will help to strengthen preparedness and response capacities in anticipation of a new pandemic by pathogen X. Novel approaches are tested on existing pathogens that bear the potential of causing major pandemics and epidemics, namely the corona virus / influenza virus / West Nile virus (Lead researcher: P. Foka, collaboration with T. Karamitros)
- Generation of Pseudotyped Viruses and VLPs for the Study of SARS-CoV-2 Entry and Endocytosis-related Immunological Events (Lead researchers: P. Foka, U. Georgopoulou)
- Development of photoactive surfaces with antiviral activity for a clean and safe environment (Lead researchers: P. Foka, U. Georgopoulou, collaboration with D. Sgouras)

- Development of a toe-hold switch-based diagnostic method for the detection of MERS-CoV (Lead researcher: P. Foka)

- HORIZON-HLTH-2022-DISEASE-07-02, Project number: 101094685 (10/2024 – 09/2027) “Integrating Multi-Disciplinary Expertise in a Learning and Adaptive European Pandemic Preparedness (LEAPS), ((P. Foka –PI and WP coordinator with T. Karamitros; E. Karamichali, U. Georgopoulou, A. Kakkanas – collaborators)
- Optima Bank Research Support donation (2023 – 2024). (U. Georogopoulou –PI)
- Cyprus Academy of Sciences, Letters and Arts (07/2023 – 12/2024), “Cognition and immunity in neurodegenerative disease: the COGNATE study” (HPI PI, Collaboration with the Medical School of the University of Cyprus) (P. Foka –PI, E. Karamichali, U. Georgopoulou – collaborators)
- Post-doctoral Fellowship from Hellenic Pasteur Institute, (07/2022 – 06/2024), “The impact of Exosome-driven immune modulation of liver disease progression following HCV eradication by DAAs” (E. Karamichali –PI, U. Georgopoulou, P. Foka - collaborators)
- Cyprus Academy of Sciences, Letters and Arts (09/2022 – 12/2023), “COVALENT: A COVID-19 Clinical, Research and Phenotyping Network, pilot study” (HPI PI, Collaboration with the Medical School of the University of Cyprus)
- State Scholarships Foundation (IKY) (2022 – 2023), “Hepatocellular carcinoma in HCV infection and the role of selected biomarkers” (U. Georgopoulou –PI)
- Regional Operational Programme "Attica" 2014-2020 9/2022, Project number: ΑΤΤΡ4-0349671– (09/2022 - 08/2024) – Development of Photoactive Surfaces with Antibacterial and Antiviral Activity for a Clean and Safe Environment “Apogeion” (U. Georgopopoulou, P. Foka – D. Sgouras-PI)
- Pharmaceutical Association of Piraeus (PEIFASYN) Donation (2022) Pilot study: The role of long COVID-19 as a trigger for molecular neurodegeneration mechanisms (P. Foka –PI)
- Gilead Sciences Hellas, Asklepios Grants (06/2022 - 05/2024): - Epigenetic regulation of the intrahepatic metastatic potential of HCV-induced Hepatocellular Carcinoma via the LSD1 – EMMPRIN axis. (P. Foka – PI, U. Georgopoulou, E. Karamichali – collaborators)
- Gilead Sciences, Hellas, Asklepios Grants (06/2022 – 05/2024), “The role of intercellular communication in the reduced immunosurveillance of HCV Chronic infection after treatment with DAAs”, (E. Karamichali-PI; P. Foka, U. Georgopoulou - collaborators)
- National Science Fund, Ministry of Education and Science, Bulgaria, Competition for financial support of basic research projects – 2021, “Coronavirus Infections - Host and Cell Interactions in the One Health Concept”, 2021-2023 (PI for HPI: U. Georgopoulou; E. Karamichali and P Foka-collaborators; Coordinator Prof. Maya Margaritova Zaharieva - S. Angeloff Institute of Microbiology, Bulgaria)
- Diagnostiki Athinon Clinical and Research Laboratory, “Development of a GC/MS-based analytical method for the determination of bioactive lipids”, 2021-2023 (P. Foka –PI in collaboration with Dr P. Eliadis - HPI)
- Nostos Donation –Hellenic Pasteur Institute competitive studentships, One-year PhD Studentship, “Hepatocellullar carcinoma in the context of viral infection and the role of selected biomarkers”, 2021-2022 (U. Georgopoulou-PI)
- Nostos Donation –Hellenic Pasteur Institute competitive studentships, Three-year PhD Studentship, “RNA Viruses and Host Immune Responses”, 2020-2023 (P. Foka -PI)
- NSRF ESPA (post-doc) “Cellular mediators of adaptive immunological response during HCV infection”, 2020-2021 (E. Karamichali-PI; U. Georgopoulou –Academic Advisor, P. Foka –Assistant Academic Advisor)
- I. Kabouris Donation: “Virus-induced Hepatocellular Carcinoma and the Role of Selective Biomarkers”, 2018-2019 (U.Georgopoulou –PI)
- Gilead Sciences, Hellas, Asklepios Grants 2019, “Exosomes and regulation of the immune response in chronic HCV infection”, 2019-2020 (E. Karamichali -PI, U. Georgopoulou, P. Foka – collaborators)
- Empeirikion Foundation, The role of exosomes as transporter of HCV genomes and miRNAs in the progression of hepatocellular cancer, 2018-2019 (U. Georgopoulou -PI)
- Gilead Sciences, Hellas, Asklepios Grants 2017, “HCV-regulated lipid metabolism of the host as a putative mechanism for HCC development”, 2018 – 2020 (P.Foka –PI; E. Karamichali and U. Georgopoulou collaborators)
- Gilead Sciences, Hellas, Asklepios Grants 2017, “Investigation of the role of exosomes as carriers of HCV viral RNA and miRNAs in HCC progression”, 2018 – 2020, (U. Georgopoulou –PI; E. Karamichali – collaborator)
- Greek General Secretariat for Research and Technology - KRHIPIS II- InfeNeuTra – MIS450598, “Infectious, autoimmune and neurodegenerative diseases: study of the pathogenic mechanisms and development of diagnostic, prognostic and therapeutic approaches”, 2017-19 (E. Karagouni - coordinator of the institutional project, U. Georgopoulou)
- Gilead Sciences, Hellas, Asklepios Grants 2016, “Investigate the role of defective genomes in HCV infection”, 2017-2019, (U. Georgopoulou –PI; E. Karamichali – collaborator)
- NFRI (ΕLΙDEK), “Cellular Signalling and Metabolic changes in HCV infection: Identification of cellular targets involved in the development/progression of HCC”, 2017-2019 (U. Georgopoulou –PI)
- Hellenic Association for the Study of the Liver, “Deregulation of Lipid Metabolism in HCC: The HCV paradigm”, 2015-2017 (U. Georgopoulou-PI, P. Foka –collaborator)
- Institut Pasteur ACIP, “The many faces of Hepatitis C virus: impact of defective genomes on pathogenesis on liver disease by assessment of exosomes secretion” 2014-2016 (U. Georgopoulou – PI; P. Pineau – Institut Pasteur Paris and S. Benjelloun – Institut Pasteur Maroc -participants)
We would like to acknowledge all funding agencies and organisations for their support

USA Patent Application Title: Nucleic Acids and new polypeptides associated with and/or overlapping with HCV core products. Mavromara P., Varaklioti A., Georgopoulou U. Patent Application No: 09/644,987
U. Georgopoulou
- Postgraduate Course of Immunology in the context of the postgraduate program ‘Clinical Biochemistry–Molecular Diagnostics’. University of Athens, Department of Biology (2013-present)
- Postgraduate Course of Immunology in the context of the postgraduate program ‘Applications of Biology in Medicine’. Medical School, University of Athens, Department of Biology (2013-2019).
- Postgraduate Course of Virology in the context of the postgraduate program in the School of Dentistry, University of Athens (2002-2012)
P. Foka
- Postgraduate Course in Applications of Biology in Medicine – Module of Immunology, Dept. of Biology, NKUA (2022)
- Postgraduate Course in Advanced Systems & Methods in Biomedical Engineering, Dept. of Biomedical Engineering, University of West Attica (2014-2022)
A. Kakkanas
- HPI “Bimonthly Health and Good Laboratory Practices Seminars” for PhD, MSc, BSc students and Interns on the following topics: Biological Risks in Laboratories. The use of GMOs. Good Laboratory Practices. Biological risks, Radiological Hazards in Laboratories, Disinfection and sterilization. Fumigation methods, Chemical Hazards in Laboratories. Good Laboratory Practices, Ergonomics and Safety in Laboratories, Other Laboratory dangers and risks.
E. Karamichali
- Lecturer in Virology course (theoretical and laboratory– 6th semester) and in Epidemiology of infectious diseases (8th semester), Dept of Biomedical sciences, University of West Attica (2023 - 2024)
- 2023: Oral Presentation Award: The epigenetic regulator LSD1 in HCV infection and its role in HCV-associated Hepatocellular carcinoma. (21st Hellenic Liver Congress, 17-20 May 2023, Ioannina, Greece – G. Papadopoulou)
- 2021: Oral Presentation Distinction: “The pivotal role of exosomal cargo in the immune response of chronic HCV patients after treatment with DAAs” (27th International Symposium on Hepatitis C Virus and Related Viruses (HCV 2021), a virtual conference, July 6 – 9, 2021 – E. Karamichali).
- 2021: Travel Award: Institut Pasteur Global Health: Viruses, Liver and Cancers Seminar (Schloss Arenberg, Salzburg, Austria, 18 – 24 July 2021 - E. Karamichali, G. Papadopoulou)
- 2019: Oral Presentation Award in the Young Scientists Category: “LSD1 as key molecule for HCV-mediated hepatic steatosis and HCC” (17th Hellenic Liver Congress, 9-11 May 2019, Kalamata, Greece – G. Papadopoulou).
- 2018: Bronze medal in the iGEM 2018 Competition: “GENOMERS: Toehold switch enabled viral detection via routine glucose monitoring technology” iGEM Athens 2018 team (Undergraduate category / Diagnostics track) (iGEM November 2018 Giant Jamboree, Boston, USA - P. Foka).
- 2018: Oral Presentation Distinction: “HCV defective genomes promote persistent infection by modulating the viral life cycle” (HCV 2018: 25th International Symposium on Hepatitis C Virus and Related Viruses, Dublin October 2018 – E. Karamichali).
- 2016: Travel Award: 3rd Institute Pasteur International Network Scientific Symposium, “From basic science to biomarkers & tools” (November 29th –December 2nd, 2016, Paris, France - E. Karamichali)
- 2015: 1st Oral Presentation Award: “Hepatitis C and cellular kinase network: An intriguing relationship” (14th Hellenic Liver Congress, Kos, Greece – P. Foka).
2024
Zaharieva MM, Foka P, Karamichali E, Kroumov AD, Philipov S, Ilieva Y, Kim TC, Podlesniy P, Manasiev Y, Kussovski V, Georgopoulou U, Najdenski HM., Photodynamic Inactivation of Bovine Coronavirus with the Photosensitizer Toluidine Blue O. Viruses 2024, 16(1), 48; doi.org/10.3390/v16010048
2023
Vavougios, GD, Mysiris, D, Foka, P, Mavridis, T, Stavrou, V, Papaggeli, O, Zarogiannis, SG, Xiromerisiou, G, Artemiadis, A, Gourgoulianis, K, Snyder, HM, de Erausquin, GA, Hadjigeorgiou, GM. SARS-CoV-2 Associated Neurocognitive Disorder (SAND): Molecular validation of a pathogenetic hypothesis. Alzheimer's Dement. 2023, 19:e077044. doi.org/10.1002/alz.077044
Papadopoulou G, Petroulia S, Karamichali E, Dimitriadis A, Marousis D, Ioannidou E, Papazafiri P, Koskinas J, Foka P, Georgopoulou U, The Epigenetic Controller Lysine-Specific Demethylase 1 (LSD1) Regulates the Outcome of Hepatitis C Viral Infection, Cells. 2023 Nov 3;12(21):2568. doi: 10.3390/cells12212568. DOI: 10.3390/cells12212568
2022
Karamichali, E, Foka, P, Valiakou, V, Eiliadis, P, Loukaki-Gkountara, D, Andresaki, K, Papadopoulou, G, Georgopoulou, U, Koskinas, IG. Exosomal cargo as a key player of the immune response after direct-acting antiviral treatment in chronic hepatitis C patients. J. Hepatol. 2022,77(S1):S255-S256. doi.org/10.3390/ijms231810862
Karamichali E, Foka P, Papadopoulou G, Loukaki-Gkountara D, Andresaki K, Koskinas I, Georgopoulou U, Hepatitis Viruses Control Host Immune Responses by Modifying the Exosomal Biogenesis Pathway and Cargo, Int J Mol Sci. 2022 Sep 17;23(18):10862. DOI: 10.3390/ijms231810862
Mysiris, DS, Vavougios, GD, Karamichali, E, Papoutsopoulou, S, Stavrou, VT, Papayianni, E, Boutlas, S, Mavridis, T, Foka, P, Zarogiannis, SG, Gourgoulianis, K, Xiromerisiou, G. Post-COVID-19 parkinsonism and Parkinson's Disease pathogenesis: The exosomal cargo hypothesis. Int. J. Mol. Sci. 2022, 23(17):9739. DOI: 10.3390/ijms23179739
Kakkanas A, Karamichali E, Koufogeorgou EI, Kotsakis SD, Georgopoulou U, Foka P., Targeting the YXXΦ Motifs of the SARS Coronaviruses 1 and 2 ORF3a Peptides by In Silico Analysis to Predict Novel Virus-Host Interactions, Biomolecules. 2022 29;12(8):1052. DOI: 10.3390/biom12081052
Georgopoulou U, Koskinas, Unraveling the Dynamic Role of Microtubules in the HBV Life Cycle, J Clin Transl Hepatol. 2022 28;10(3):383-385. DOI: 10.14218/JCTH.2022.00135
2021
Foka P, Dimitriadis A, Karamichali E, Kochlios E, Eliadis P, Valiakou V, Koskinas J, Mamalaki A, Georgopoulou U. HCV-Induced Immunometabolic Crosstalk in a Triple-Cell Co-Culture Model Capable of Simulating Systemic Iron Homeostasis. Cells 2021, 10(9):2251DOI: 10.3390/cells10092251
Valiakou V, Eliadis P, Karamichali E, Tsitsilonis O, Koskinas J, Georgopoulou U, Foka P. Differential Expression of the Host Lipid Regulators ANGPTL-3 and ANGPTL-4 in HCV Infection and Treatment. Int J Mol Sci. 2021, 22(15):7961. DOI: 10.3390/ijms22157961
Dimitriadis A., Foka P., Kyratzopoulou E., Karamichali E., Petroulia S., Tsitoura P., Kakkanas A., Eliadis P., Georgopoulou U., Mamalaki A. The Hepatitis C Virus NS5A and core proteins exert antagonistic effects on HAMP gene expression: the hidden interplay with the MTF-1/MRE pathway. FEBS Open Bio 2021, 11(1):237-250. DOI: 10.1002/2211-5463.13048
Mourtzi N, Siahanidou T, Tsifintaris M, Karamichali E*, Tasiopoulou A, Sertedaki A, Pesmatzoglou M, Kapetanaki A, Liosis G, Baltatzis G, Vlachakis D, Chrousos GP, Giannakakis A. lncRNA NORAD is consistently detected in breastmilk exosomes and its expression is downregulated in mothers of preterm infants. Int J Mol Med. 2021, 48(6):216. DOI: 10.3892/ijmm.2021.5049
2018
Karamichali E., Chihab H., Kakkanas A., Marchio A., Karamitros T., Pogka V., Varaklioti A., Kalliaropoulos A., Martinez-Gonzales B., Foka P., Koskinas I., Mentis A., Benjelloun S., Pineau P., Georgopoulou U. (2018). HCV Defective Genomes Promote Persistent Infection by Modulating the Viral Life Cycle. Front. Microbiol. 9:2942. DOI: 10.3389/fmicb.2018.02942
Chihab H, Jadid FZ, Foka P, Zaidane I, El Fihry R, Georgopoulou U, Marchio A, Elhabazi A, Chair M, Pineau P, Ezzikouri S, Benjelloun S, (2018). Programmed cell death-1 3'-untranslated region polymorphism is associated with spontaneous clearance of hepatitis B virus infection. J Med Virol. 2018 Jul 17. doi: 10.1002/jmv.25265. DOI: 10.1002/jmv.25265
Molyvdas A, Georgopoulou U, Lazaridis N, Hytiroglou P, Dimitriadis A, Foka P, Vassiliadis T, Loli G, Phillipidis A, Zebekakis P, Germenis AE, Speletas M, Germanidis G., (2018). The role of the NLRP3 inflammasome and the activation of IL-1β in the pathogenesis of chronic viral hepatic inflammation, Cytokine. May 23. pii: S1043-4666(18)30185-6. DOI: 10.1016/j.cyto.2018.04.032
Karamitros T, Papatheodoridis G, Paraskevis D, Hatzakis A, Mbisa JL, Georgopoulou U, Klenerman P, Magiorkinis G., (2018). Impact of Interferon-α Receptor-1 Promoter Polymorphisms on the Transcriptome of the Hepatitis B Virus-Associated Hepatocellular Carcinoma. Front Immunol. Apr 16;9:777. DOI: 10.3389/fimmu.2018.00777
Aicher S, Kakkanas, A, Cohen L, Blumen B , Oprisan G, Njouom R, Meurs EF, Mavromara P, Martin A, (2018). Differential regulation of the Wnt/β-catenin pathway by hepatitis C virus recombinants expressing core from various genotypes. Sci Rep. 8(1):11185. DOI: 10.1038/s41598-018-29078-2
2017
Karamichali E, Serti A, Gianneli A, Papaefthymiou A, Kakkanas A, Foka P, Seremetakis A, Katsarou K, Trougakos IP, Georgopoulou U, (2017). The unexpected function of a highly conserved YXXΦ motif in HCV core protein, Infect Genet Evol. 54:251-262. DOI: 10.1016/j.meegid.2017.07.001
Kaffe E, Katsifa A, Xylourgidis N, Ninou I, Zannikou M, Harokopos V, Foka P, Dimitriadis A, Evangelou K, Moulas A, Georgopoulou U, Gorgoulis V, Dalekos G, Aidinis V, (2017). Hepatocyte Autotaxin expression promotes liver fibrosis and cancer, Hepatology 65(4):1369-1383. DOI: 10.1002/hep.28973
Dalagiorgou G, Piperi C, Adamopoulos C, Georgopoulou U, Gargalionis AN, Spyropoulou A, Zoi I, Nokhbehsaim M, Damanaki A, Deschneer J, Basdra EK, Papavassiliou AG, (2017). Mechanosensor polycystin-1 potentiates differentiation of human osteoblastic cells by upregulating Runx2 expression via induction of JAK2/STAT3 signaling axis Cell Mol Life Sci. 74(5):921-936. DOI: 10.1007/s00018-016-2394-8
2016
Foka P, Dimitriadis A, Karamichali E, Kyratzopoulou E, Giannimaras D, Koskinas J, Varaklioti A, Mamalaki A, Georgopoulou U, (2016). Alterations in the iron homeostasis network: A driving force for macrophage-mediated hepatitis C virus persistency Virulence. 7:679-90. DOI: 10.1080/21505594.2016.1175700
Karamouzis MV, Dalagiorgou G, Georgopoulou U, Nonni A, Kontos M, Papavassiliou AG, (2016). HER-3 targeting alters the dimerization pattern of ErbB protein family members in breast carcinomas. Oncotarget 7:5576-5597. DOI: 10.18632/oncotarget.6762
2015
Doumba PP, Serti E, Boutsikou M, Konstandoulakis MM, Georgopoulou U, Koskinas J, (2015). The role of caveolin-1 in the phenotype of human t lymphocytes after uptake of hcv non-enveloped particles. J Hepatol. 62:S579-S579.
Foka P, Dimitriadis A, Karamichali E, Kyratzopoulou E, Giannimaras D, Koskinas J, Mamalaki A, Georgopoulou U, (2015). Regulation of hepcidin (hamp) as driving force for macrophage-mediated hepatitis c (hcv) persistency. J Hepatol 62:S579-S580.
Simos T, Georgopoulou U, Thyphronitis G, Koskinas J, Papaloukas C, (2015). Analysis of Protein Interaction Networks for the Detection of Candidate Hepatitis B and C Biomarkers. IEEE J Biomed Health Inform. 19:181-189. DOI: 10.1109/JBHI.2014.2344732
Selected Publications 2000 - 2014
Foka P, Karamichali E, Dalagiorgou G, Serti E, Doumba PP, Pissas G, Kakkanas A, Kazazi D, Kochlios E, Gaitanou M, Koskinas J, Georgopoulou U, Mavromara P, (2014). Hepatitis C virus modulates lipid regulatory factor Angiopoietin-like 3 gene expression by repressing HNF-1 alpha activity. J Hepatol. 60:30-38. DOI: 10.1016/j.jhep.2013.08.016
Foka P, Dimitriadis A, Kyratzopoulou E, Giannimaras DA, Sarno S, Simos G, Georgopoulou U, Mamalaki A, (2014). A complex signaling network involving protein kinase CK2 is required for hepatitis C virus core protein-mediated modulation of the iron-regulatory hepcidin gene expression.Cell Mol Life Sci. 71(21):4243-58. DOI: 10.1007/s00018-014-1621-4
Georgopoulou U, Dimitriadis A, Foka P, Karamichali E, Mamalaki A, (2014). Hepcidin and the iron enigma in HCV infection. Virulence 5:465-476. DOI: 10.4161/viru.28508
Karamichali E, Foka P, Tsitoura E, Kalliampakou K, Kazazi D, Karayiannis P, Georgopoulou U, Mavromara P, (2014). HCV NS5A co-operates with PKR in modulating HCV IRES-dependent translation. Infect. Genet. Evol. 26:113-122. DOI: 10.1016/j.meegid.2014.04.015
Serti E, Doumba PP, Thyphronitis G, Tsitoura P, Katsarou K, Foka P, Konstadoulakis MM, Koskinas J, Mavromara P, Georgopoulou U, (2011). Modulation of IL-2 expression after uptake of hepatitis C virus non-enveloped capsid-like particles: the role of p38 kinase. Cell Mol Life Sci. 68(3):505-522. DOI: 10.1007/s00018-010-0466-8
Katsarou K, Tsitoura P, Georgopoulou U, (2011). MEK5/ERK5/mef2: a novel signaling pathway affected by hepatitis C virus non-enveloped capsid-like particles Biochim Biophys Acta 1813(10):1854-62. DOI: 10.1016/j.bbamcr.2011.06.015
Katsarou K, Lavdas AA, Tsitoura P, Serti E, Markoulatos P, Mavromara, Georgopoulou U (2010). Endocytosis of hepatitis C virus non-enveloped capsid-like particles induces MAPK-ERK1/2 signalling events. Cell Mol Life Sci 67(14):2491-2506. DOI: 10.1007/s00018-010-0351-5
Foka P, Pourchet A, Hernandez-Alcoceba R, Doumba PP, Pissas G, Kouvatsis V, Dalagiorgou G, Kazazi D, Marconi P, Foschini M, Manservigi R, Konstadoulakis MM, Koskinas J, Epstein AL, Mavromara P, (2010). Novel tumour-specific promoters for transcriptional targeting of hepatocellular carcinoma by herpes simplex virus vectors. J Gene Med. 12(12):956-67. DOI: 10.1002/jgm.1519
Katsarou K, Serti E, Tsitoura P, Lavdas AA, Varaklioti A, Pickl-Herk AM, Blaas D, Oz-Arslan D, Zhu R, Hinterdorfer P, Mavromara P, Georgopoulou U, (2009). Green fluorescent protein - Tagged HCV non-enveloped capsid like particles: Development of a new tool for tracking HCV core uptake. Biochimie 91(7):903-915. DOI: 10.1016/j.biochi.2009.04.016
Tsitoura P, Georgopoulou U, Pêtres S, Varaklioti A, Karafoulidou A, Vagena D, Politis C, Mavromara P, (2007). Evidence for cellular uptake of recombinant hepatitis C virus non-enveloped capsid-like particles FEBS Lett. 581(21):4049-57. DOI: 10.1016/j.febslet.2007.07.032
Georgopoulou U, Tsitoura P, Kalamvoki M, Mavromara P, (2006). The protein phosphatase 2A represents a novel cellular target for hepatitis C virus NS5A protein. Biochimie 88(6):651-662. DOI: 10.1016/j.biochi.2005.12.003
Varaklioti A, Vassilaki N, Georgopoulou U, Mavromara P, (2002). Alternate translation occurs within the core coding region of the hepatitis C viral genome. J Biol Chem. 277(20):17713-17721. DOI: 10.1074/jbc.M201722200








Papadopoulou Georgia
Mysiris Dimitrios
• Athanassios Batsilas (2022-present) – Dept. of Biology, NKUA
• Morakou Maria (2023- present) - Dept. of Biomedical Sciences, University of West Attica
• Christina Varthali (2023 – present) – Dept. of Biology, NKUA
• Hajar Chihab (Visiting PhD student – 2015)
• Sofia Mazi (MSc thesis – 2024)
• Stavroula Petroulia, (MSc thesis - 2019)
• Vasiliki Petroulaki (MSc thesis – 2016)
• Despoina Olga Papaggeli, (Degree thesis – 2023)
• Dimitrios Maroussis, (Degree thesis – 2023)
• Alfonsos – Eleftherios Delis (Degree thesis – 2021)
• Konstantina Andresaki, (Degree thesis - 2020)
• Domniki Loukaki Gkountara, (Degree thesis - 2020)
• Georgios Anastasiou, (Degree thesis - 2019)
• Elisavet Ioannidou, (Degree thesis – 2019)
• Maria Litsa, (Degree thesis – 2019)
• Alexandros Seremetakis (Degree thesis – 2016)
• Dionyssia Marinou (Degree thesis – 2015)
• Natalia Bartzoka (Internship – 2019)
• Eleni Kostadima (Internship – 2019)
• Dimitris Arvanitis (Internship – 2017)
Prof. Ourania Tsitsilonis, Professor of Immunology, Dept. of Biology, NKUA
Prof. Aikaterini Gaitanaki, Professor of Physiology, Dept. of Biology, NKUA
Dr Panagiota Papazafeiri, Associate Professor of Physiology, Dept. of Biology, NKUA
Dr Evaggelos Topakas, Associate Professor of Biotechnology, School of Chemical Engineering NTUA
Prof. Konstantinos Gourgoulianis, Professor of Pulmonology, School of Medicine, University of Thessaly
Dr Georgia Xiromerisiou, Ass. Professor of Neurology, School of Medicine, University of Thessaly
Prof. George Hatzigeorgiou, Professor of Neurology, School of Medicine, University of Cyprus.
Dr George D. Vavougios, Lecturer of Neurology, School f Medicine, University of Cyprus.
Theodore Mavridis, Consultant Neurologist/Stroke Specialist, Tallaght University Hospital Ireland
Prof. Hristo M. Najdenski, Professor of Microbiology, The Stephan Angeloff Institute of Microbiology, Bulgaria
Dr. Maya Zaharieva, Ass. Professor, The Stephan Angeloff Institute of Microbiology, Bulgaria
Dr Vassilis Aidinis, Research director, Fleming BSRC
Dr Georgios Germanidis, Associate Professor of Gastroenterology, Dept. of Medicine, AUTH
Dr Antonios Giannakakis, Assistant Professor of Computational Biology, Dept. of Molecular Biology and Genetics DUT
Prof. Georgios Thyphronitis, Professor of Immunology, Dept. of Biological Applications and Technology, UOI
Dr Kostas Papaloukas, Associate Professor of Bioinformatics, Dept. of Biological Applications and Technology, UOI
Dr Theologos Michaelidis, Associate Professor of Molecular Genetics, Dept. of Biological Applications and Technology, UOI
Prof. Haralambos Stamatis, Professor of Enzyme Technology, Dept. of Biological Applications and Technology, UOI
Dr Agoritsa Varaklioti, Centre for Congenital Bleeding Disorders, Laiko General Hospital
Dr Konstantina Katsarou, Post-doctoral Researcher, Plant Molecular Biology Laboratory, IMBB
Dr Soumaya Benjelloun, Principal Investigator, Viral Hepatitis Laboratory, Institut Pasteur du Maroc,
Dr Pascal Pineau, Researcher, INSERM U993, Unité "Organisation Nucléaire et Oncogenèse", Institut Pasteur Paris
Dr George Bertsias, Ass. Professor of Rheumatology and Clinical Immunology, School of Medicine, University of Crete
Dr Charalambos Pontikoglou, Ass. Professor of Haematology, School of Medicine, University of Crete
Dr Alexandra Voutsina, Researcher Grade C’, National Hellenic Research Foundation
